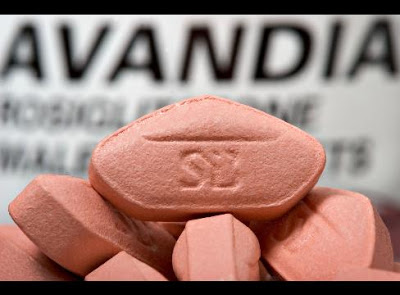
It has been an absolute whirlwind week at the European Association for the Study of Diabetes Meeting in Stockholm, Sweden, and I have many interesting thing to share with my readers over a series of upcoming blogs. The week’s biggest news is pretty much uncontested, however, and it regards the future of a commonly used diabetes medication called rosiglitazone, or Avandia.
Both the European Medicines Agency (EMEA) and the American FDA released statements regarding the future of Avandia on September 23, 2010. While the EMEA has suspended the use of Avandia in Europe, the FDA has allowed Avandia use to continue, while restricting access and adding additional safety labeling.
Avandia has been under mounting scrutiny since a meta analysis of data suggested that Avandia use may be associated with an increase risk of cardiovascular events. By controlling blood sugars, one of the major complications of diabetes that we are aiming to PREVENT, of course, is heart disease. While more than one such analysis of data has suggested there may be an increased cardiac risk with Avandia, no ‘gold standard’ randomized, controlled, clinical trial has proven this to be the case. The RECORD trial, which was a randomized clinical trial, did not demonstrate a significantly increased cardiovascular risk (though this data is subject to several criticisms).
With these analyses in hand, then, regulatory agencies have had a very difficult and controversial decision to make, and it is due to this uncertainty that the EMEA and the FDA have leaned in slightly different directions this week.
The European Medicines Agency concluded that the benefits of rosiglitazone no longer outweigh its potential risks. They indicate that Avandia will no longer be available in Europe in a few months’ time, and advise patients to book an appointment with their physician to plan cessation of Avandia and to discuss other suitable medication to treat their diabetes. The suspension will remain in place unless convincing data can be provided to identify a group of patients in whom the benefits of Avandia outweigh their risks.
The FDA, on the other hand, has allowed Avandia use to continue, but has restricted its use to those who cannot control their diabetes on other medications. The FDA will also require a Risk Evaluation and Mitigation Strategy program with additional measures to ensure the safe use of the medicine. This includes a reevaluation of data from the RECORD trial mentioned above.
In addition, the FDA has put the TIDE trial on hold, which was designed to compare Avandia to a drug in the same class called Actos, with regard to effect on cardiovascular outcomes in high risk patients with type 2 diabetes.
For any patients who are taking Avandia and are either affected by it suspension (ie, living in Europe) or unsure of how to proceed, be sure to book in with your doctor to discuss before making any changes.
Follow me on Twitter for more tips! drsuepedersen