Prefer to listen? Check out my podcast here!
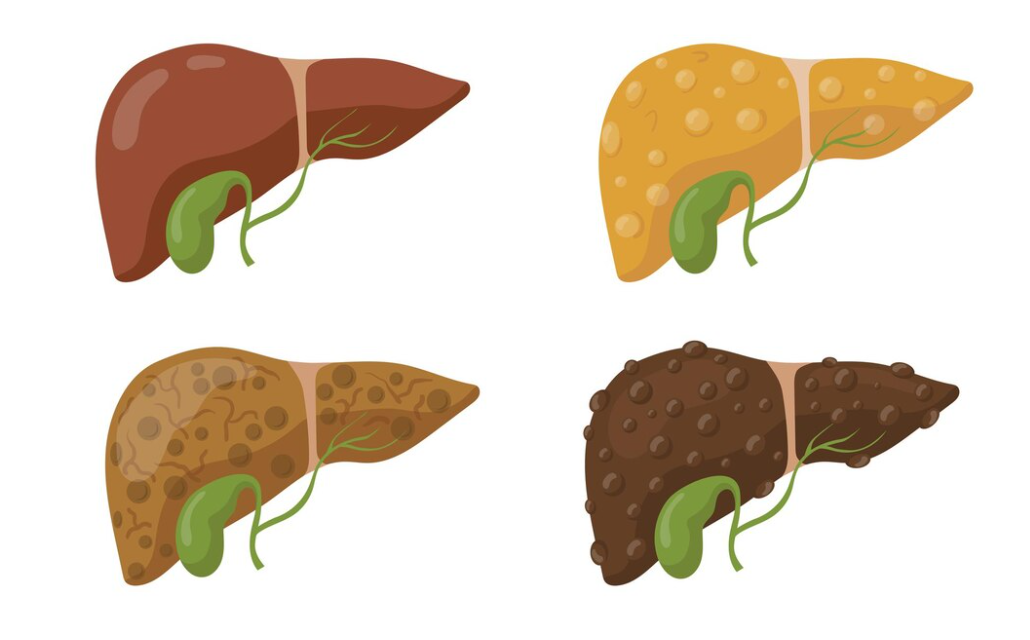
Fatty liver disease is very common, affecting about 70% of people with obesity or type 2 diabetes. Resmetirom (trade name Rezdiffra), a thyroid hormone receptor beta-selective agonist, is the first medication ever approved to treat fatty liver disease (approved a few days ago by the American FDA). Many other treatments are being investigated for fatty liver disease, including GLP1-based medications that are approved or in develoment for obesity and/or diabetes.
Resmetirom is currently under investigation in the MAESTRO-NASH phase 3 trial, with the one year results recently published in the New England Journal of Medicine. They included 966 people with biopsy confirmed NASH (non alcoholic steatohepatitis, recently renamed as MASH, metabolic dysfunction-associated steatohepatitis) and a fibrosis (liver scarring) stage of F1B, F2, or F3, to receive a daily tablet of resmetirom 80mg, 100mg, or placebo.
At one year, resolution of NASH with no worsening of fibrosis was achieved in 25.9% of people with 80mg resmetirom, 29.9% with 100mg resmetirom, vs 9.7% with placebo. Fibrosis improvement by at least one stage with no worsening of the NAFLD (non alcoholic fatty liver disease, now called MAFLD or metabolic-dysfunction associated fatty liver disease) activity score was achieved in 24% and 25.9% of the resmetirom 80mg and 100mg groups respectively, vs 14.2% of those in the placebo group. Note that there is no change in weight with resmetirom.
We know that weight loss can improve fatty liver disease (more on this blogged here). In our Obesity Canada Clinical Practice Guidelines pharmacotherapy (medications) chapter, we systematically searched the literature to explore whether obesity medications can improve fatty liver disease (disclosure: I am the lead author). The GLP1 receptor agonists liraglutide (trade names Saxenda for obesity, Victoza for diabetes) and semaglutide (trade names Wegovy for obesity, Ozempic for diabetes) have demonstrated that they can improve NASH/MASH parameters, and we therefore recommend consideration of these medications for treatment of people with obesity and NASH/MASH. That being said, neither medication is formally approved as a fatty liver disease treatment.
The hot ticket with resmetirom (and why it is now approved in the US as a fatty liver disease treatment) is that it results in fibrosis (scarring) improvement. Fibrosis severity is the only histological (biopsy) predictor of liver-related morbidity (illness) and mortality in NASH/MASH, and fibrosis regression is associated with improved clinical outcomes. While there are data supporting improvement in fibrosis with diabetes medication pioglitazone (Actos) in people with type 2 diabetes and fatty liver disease, it is not commonly prescribed due to side effect concerns (including weight gain). Vitamin E may be of benefit in people with fatty liver disease without diabetes, but some observational studies suggest higher mortality with higher doses. Other than that, no published data on any other medication have shown improvement in fibrosis. But that may change soon.
A recent investor report revealed that tirzepatide, a dual GIP/GLP1 receptor agonist (trade names Zepbound for obesity, Mounjaro for diabetes), resulted in 73.9% absence of MASH with no worsening of liver histology with the highest dose of tirzepatide (15mg weekly) compared to 12.6% with placebo in a phase 2 trial of people with biopsy-proven MASH with stage 2 or 3 fibrosis. And – the secondary endpoint of decrease in fibrosis by at least one stage with no worsening of MASH on liver histology was ‘clinically meaningful’ across doses. Tirzepatide can provide powerful weight loss as blogged previously; we don’t yet know how much weight was lost in this MASH trial.
A high-level results release of a phase 2 study of survodutide, a dual GLP1/glucagon receptor agonist, reported that up to 83% of participants treated with survodutide experienced improvement in MASH vs 18.2% with placebo. The study met its primary endpoint of reaching a biopsy-proven improvement in MASH after 48 weeks, without worsening of fibrosis stages F1, F2, and F3 (mild to moderate or advanced scarring). Survodutide also met the secondary endpoint of a statistically significant improvement in fibrosis. Survodutide is also being evaluated as an obesity treatment (recent results blogged here), but data on the weight loss in this MASH study is not yet available.
Neither the tirzepatide nor the survodutide data have been presented nor published yet, and I certainly look forward to evaluating these results in detail. We should not compare results of one trial to another directly… but it is hard not to notice that numerically, the results for tirzepatide and survodutide look much more impressive than those for resmetirom. We don’t yet know just how much improvement in fibrosis was seen with survodutide nor tirzepatide, and those data will be very important in understanding the potential benefits of these treatments to NASH/MASH. But given that GLP1 based medications may have the potential to improve fatty liver while holistically improving health by also improving weight, it seems that these treatments may become very important in NASH/MASH treatment in the future.
BOTTOM LINE: The approval of resmetirom as the first medication for fatty liver disease is a game-changer in the field, and very exciting. I look forward to learning more about other new potential treatments as the evidence unfolds!
Disclaimer: I receive honoraria as a continuing medical education speaker and consultant from the maker of semaglutide and liraglutide (Novo Nordisk), tirzepatide (Eli Lilly), and survodutide (Boehringer Ingelheim). I am/have been an investigator in clinical trials of semaglutide, liraglutide, tirzepatide, and survodutide.
Check me out on X/twitter! @drsuepedersen
Share this blog post using your favorite social media link below!
www.drsue.ca © 2024