Prefer to listen? Check out my podcast here:
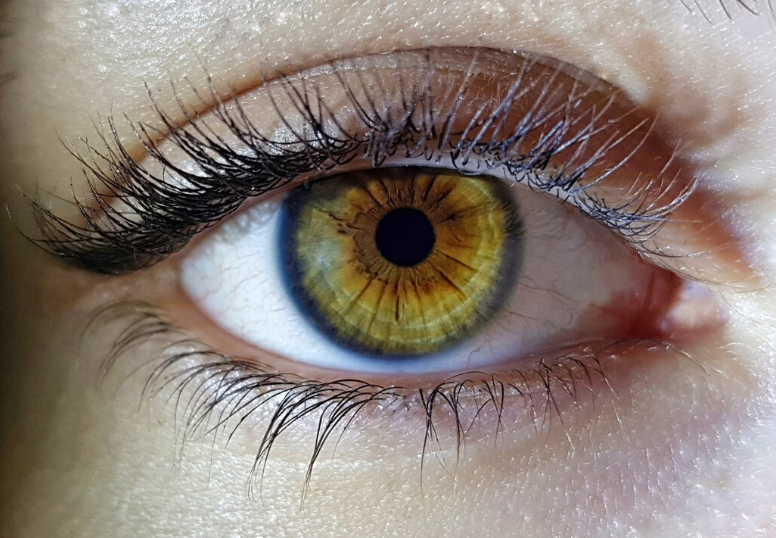
A study just published in JAMA Opthalmology has been a big news topic this week, suggesting an association between semaglutide (Ozempic or Rybelsus for diabetes, Wegovy for weight loss) and nonarteritic anterior ischemic optic neuropathy (NAION), which can cause blindness. What’s the scoop?
First of all, what is NAION? NAION is an extremely rare swelling of the optic nerve (the nerve responsible for vision), thought to be caused by decreased blood flow to the nerve. Risk factors for NAION include older age, diabetes, high blood pressure, sleep apnea, and kidney failure. NAION is very rare, occuring in 2-10 per 100,000 people per year in the general population. People who have NAION present with loss of vision, usually in one eye, occurring over the course of hours to days.
The authors looked retrospectively at data from a registry of one opthalmology (eye specialty) clinic at Harvard from 2017-2023. They identified cases of NAION in their database, and identified who was prescribed and dispensed semaglutide and who was not. They propensity matched patients (to try to minimize confounding effects) for gender, age, hypertension, type 2 diabetes, obstructive sleep apnea, coronary artery disease, high cholesterol, and use of other medications known to carry an increased NAION risk. They evaluated groups prescribed semaglutide for type 2 diabetes and obesity separately.
Amongst 710 people with type 2 diabetes, they found that the risk of NAION over 36 months was 8.9% in the semaglutide group, vs 1.8% for people not taking semaglutide (but taking other non GLP1 medications for diabetes). The risk for developing NAION was 4.3 times higher in the semaglutide group. Amongst 979 people with obesity taking medication to treat their obesity, the found the risk of NAION over 36 months to be 6.7% in the semaglutide group vs 0.8% for the nonsemaglutide group. The risk for developing NAION was 7.6 times higher in the semaglutide group. If NAION was to occur, it was more likely within the first year of semaglutide being prescribed.
At first glance, the percentage of people who had NAION sounds scary – but remember, the group studied was an opthalmology specialty clinic, not the general population as a whole. As NAION in the general population is rare, if there is an increased risk of NAION, it would still be a very low risk with semaglutide.
We always have to intepret retrospective database analyses cautiously, as they are inherently limited by potential confounding factors. I noticed in their analysis, for example, that they did not match for body mass index (BMI), nor for duration of diabetes, which could potentially have an impact on the risk of NAOIN. We also don’t know if people were actually taking semaglutide when the NAOIN took place. The gold standard for evaluation of medications is with randomized controlled clinical trials. Amongst over 25,000 people studied in randomized controlled trials of semaglutide, NAION did not emerge as a potential concern.
The above being said, while this study (by its retrospective database nature) cannot establish causality, it is possible that this study identifies a new safety concern. Further evaluation in larger and broader populations, and ongoing vigilance and reporting of potential NAOIN cases, are important.
Disclaimer: I receive honoraria as a continuing medical education speaker and consultant from the maker of semaglutide (Novo Nordisk). I am/have been an investigator in clinical trials of semaglutide.
Check me out on X/twitter! @drsuepedersen
Share this blog post using your favorite social media link below!
www.drsue.ca © 2024