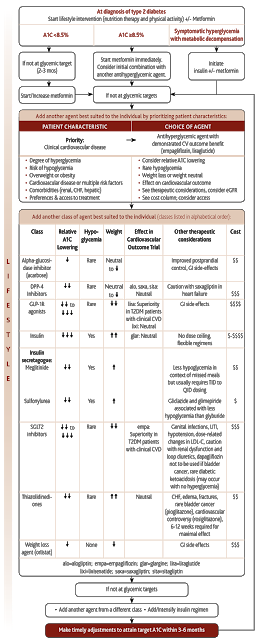
While the full Canadian Diabetes Association (CDA) Clinical Practice Guidelines are formally updated every 5 years (with the next edition due in 2018), interim updates are published if new evidence emerges that is considered to be practice changing. As such, the CDA has just released an interim update with revised recommendations, in light of the new cardiovascular outcome trial of a diabetes medication called liraglutide.
As blogged previously, in people with type 2 diabetes who were at high risk of cardiovascular disease, the liraglutide cardiovascular trial (called the LEADER trial) demonstrated that liraglutide reduced the risk of cardiovascular events by 13%. Put another way: if 66 people are treated for 3 years with liraglutide, one cardiovascular event would be prevented.
In the LEADER trial, 81% of patients had a past history of established cardiovascular disease, while 19% of patients did not (but they were still considered to be at high risk of CV events due to their risk factors). Subgroup analyses suggested that it was patients who had a history of established cardiovascular disease who had the reduction in risk with liraglutide. As patients had to be age 50 or older to be included in the study, we do not know if these findings apply to a younger population.
In the revised CDA Guidelines, liraglutide now joins another medication called empagliflozin, as medications to consider after metformin, in patients with type 2 diabetes and established cardiovascular disease, who are not at target blood sugar control. As ongoing cardiovascular outcome trials of diabetes medications are completed and published, the CDA Guidelines will be updated accordingly.
I have pasted the new algorithm below, but the resolution isn’t great – it’s a little friendlier on the eyes here.
Disclaimer: I am a member of the Expert Committee for the writing of the Canadian Diabetes Association 2018 Clinical Practice Guidelines.